Formation of nucleobases in a Miller–Urey reducing atmosphere
The study shows that Miller–Urey experiments produce RNA nucleobases in discharges and laser-driven plasma impact simulations carried out in a simple prototype of reducing atmosphere containing ammonia and carbon monoxide. We carried out a self-standing description of chemistry relevant to hypothesis of abiotic synthesis of RNA nucleobases related to early-Earth chemical evolution under reducing conditions. The research addresses the chemistry of simple-model reducing atmosphere (NH3 + CO + H2O) and the role of formamide as an intermediate of nucleobase formation in Miller–Urey experiment. The explorations combine experiments performed using modern techniques of large, high-power shock wave plasma generation by hall terawatt lasers, electric discharges, and state-of-the-art ab initio free-energy calculations.
Abstract
The Miller–Urey experiments pioneered modern research on the molecular origins of life, but their actual relevance in this field was later questioned because the gas mixture used in their research is considered too reducing with respect to the most accepted hypotheses for the conditions on primordial Earth. In particular, the production of only amino acids has been taken as evidence of the limited relevance of the results. Here, we report an experimental work, combined with state-of-the-art computational methods, in which both electric discharge and laser-driven plasma impact simulations were carried out in a reducing atmosphere containing NH3 + CO. We show that RNA nucleobases are synthesized in these experiments, strongly supporting the possibility of the emergence of biologically relevant molecules in a reducing atmosphere. The reconstructed synthetic pathways indicate that small radicals and formamide play a crucial role, in agreement with a number of recent experimental and theoretical results.
More than 60 y ago, Stanley Lloyd Miller and Harold Clayton Urey, in their pioneering work (1), demonstrated the synthesis of several amino acids from a mixture of reducing gases (CH4, NH3, H2O, and H2) treated with electric discharge. The following explorations showed that a broad array of amino acids could be synthesized, but there was no evidence that all of the fundamental molecules of the RNA genetic code could be produced alongside others in this type of experiment (2⇓⇓–5). Additionally, the significant persistence of reducing atmospheres in a geological timescale has been seriously debated (6). Finally, many scientists have claimed that this experiment is not related to early-Earth conditions and does not provide fundamental building blocks (i.e., nucleobases) important for the evolution of early life possibly based on RNA (7⇓⇓⇓⇓⇓–13). In 2001, Saladino, Di Mauro, and coworkers (14) proposed that the parent molecule for the one-pot synthesis of nucleobases is formamide (15⇓⇓⇓⇓⇓⇓⇓–23). Their team, together with other authors, demonstrated the formation of (not only) fundamental nucleobases for the origin of RNA in experiments involving the heating of formamide in presence of manifold catalysts (17, 24⇓–26), upon UV irradiation (27), proton (28) and heavy-particle radiation (29), exposition to shock waves (18), etc. Recently, Hörst et al. (30) also referred to a positive result on qualitative detection of RNA nucleobases and manifold amino acids from tholines created in a N2, CH4, CO mixture. Their experiment simulated the atmosphere of Titan upon electric discharge. Such experimental results as well as theoretical expectations (31) show that reduced, relatively reactive atmospheres are likely to be more efficient for the synthesis of biomolecules (32). However, it should be noted that several papers report also the formation of biomolecules under neutral (N2, CO2, H2O) conditions (33⇓–35). In our study, we found an interconnection between the original ideas of the pioneering Miller–Urey studies devoted to prebiotic synthesis in a reducing atmosphere and recent results identifying the chemistry of formamide as a source for the synthesis of nucleobases. In addition to traditional hydrogen cyanide (HCN)-based or reducing atmosphere-based concepts of biomolecule formation (30, 36⇓–38), we show that, under the conditions demonstrated in this study, the formamide molecule not only plays the role of parent compound but also is an intermediate in a series of reactions leading from very reactive and rigid small radicals to biomolecules.
During the past decade, comets (39), HCN hydrolysis (40), chemistry in interstellar space (41), reducing atmospheres (42), or ammonium formate dehydration (43) have been proposed as sources of formamide. However, in most cases, the exact chemistry of such systems has not been well explored either experimentally or theoretically. Moreover, the plausibility and relation to a prebiotic environment is also questioned.
Using the large laser facility at the terrawatt Prague Asterix Laser System, we comprehensively explored asteroid shock wave impact plasma, in addition to electric discharge, in a simple reducing mixture of NH3 + CO and H2O. The formamide molecule does not directly play the role of starting substrate, but it is rather a suspected intermediate of reactions leading from simple model prebiotic mixtures to biomolecules.
The results are compared with similar experiments, in which formamide is the starting compound. The chemistry is also mapped using state-of-the-art ab initio molecular-dynamics simulations. We focused our effort on two environments relevant to prebiotic chemistry: (i) transformation of an atmosphere exposed a shock wave induced by an extraterrestrial body and the resulting impact plasma [Early and Late Heavy Bombardment in our solar system during evolution and stabilization of orbits (44)]; and (ii) transformation of an atmosphere by an electrical discharge (lightning in heavy clouds of dust, vapors, and other aerosols from impact, volcanic activity and evaporation in the early atmosphere) (45).
Recently, Saitta and Saija published (42) a computer-simulated Miller-type experiment via ab initio calculations that included external electric fields. In that study, the authors show in atomistic computer simulations that an initial mixture of the simple Miller molecules (CH4, NH3, H2O, CO, N2) (1, 46, 47) spontaneously produces, on a picosecond timescale, small intermediate organic compounds, such as formic acid and formamide, but only in strong electric fields. Formamide, in particular, was continuously produced and fueled the formation of more complex organic molecules up to the simplest amino acid, glycine. Subsequently, a theoretical study on formamide synthesis and dissociation chemistry pointed to the importance of HCN, NH3, CO, and H2O in its reaction network (48).
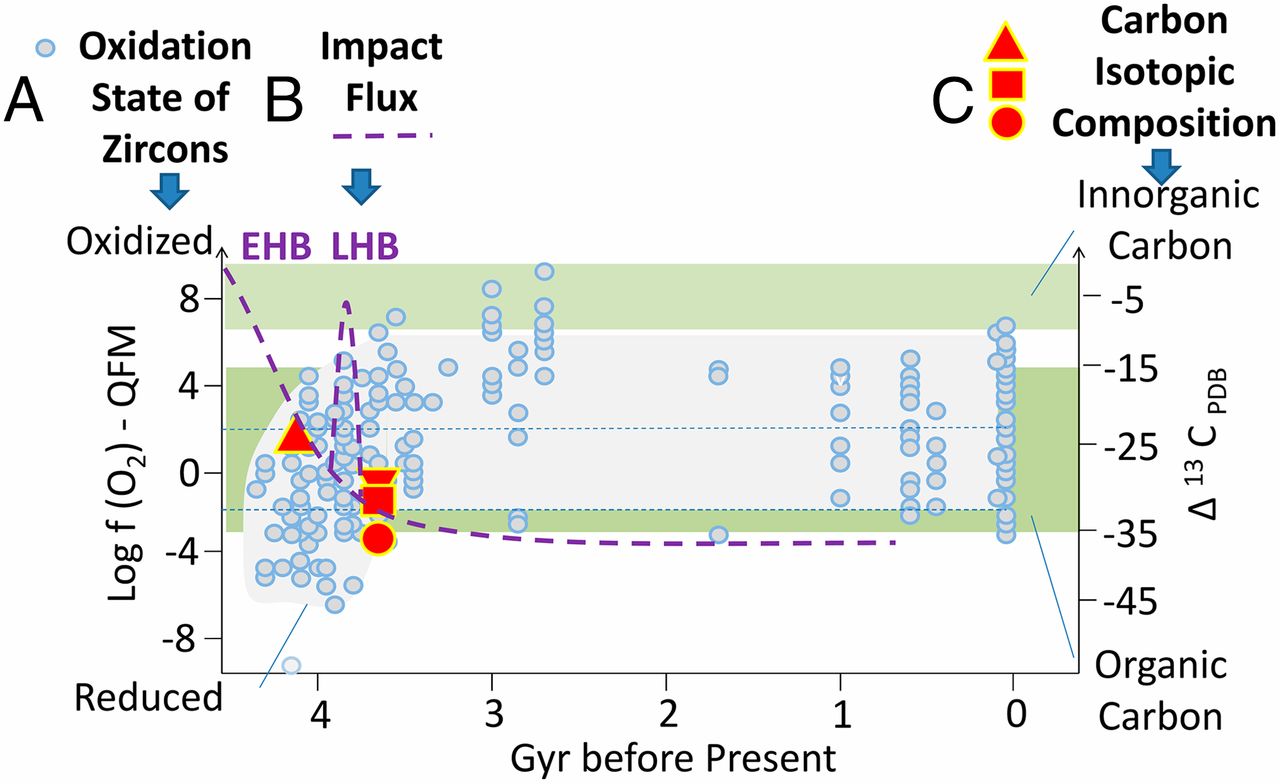
The existence of a global reducing atmosphere on early Earth has been debated during the almost 60 y after the famous Miller–Urey experiment (22). So far, the most accepted theories are inclined toward a neutral atmosphere containing an excess of CO2 with N2 and water vapor (6, 49, 50). In contrast to previous studies, a neutral atmosphere has also been identified as a plausible environment for the synthesis of amino acids (33). The existence of a neutral atmosphere was additionally supported by the first examination of the oxidation state of igneous detrital zircons related to magma before 3.85 Gy (51). However, recent results (52) of further trace element analysis of igneous zircon of crustal origin show that the Hadean continental crust was probably more reduced than its modern counterpart and that it oxidized 3.6 billion years ago, as shown in Fig. 1, part A (blue circles).
Fig. 1.
Data published in ref. 52 on the oxidation state of trace elements in early zircons (part A, blue circles), which are compared with the timescale of impact flux on early Earth (part B, violet curve; EHB and LHB are Early and Late Heavy Bombardment periods), and the age of carbon inclusions exhibiting 13C deficiency consistent with their biogenic origin (part C, red points). Data from refs. 19, 52, 56, and 67, and references therein.
It can be assumed that the low stability of reducing atmospheres was counteracted by dynamical effects, such as the exogenous transfer of reducing gases by comets (32) and chondrites (53) or by continuous processes of endogenous synthesis (31, 54). In particular, there is an interesting coincidence in the comparison of the timescales of reducing conditions, which were estimated using the analysis of zircons, as mentioned above (52) and shown in Fig. 1, part A, and the timescales of Early and Late Heavy Bombardment periods, depicted schematically in the violet curve imprinted in these data (Fig. 1, part B). We can assume that the impact-degassed atmosphere led to a reduced chemical composition (e.g., H2, CO, CH4, and NH3) (55). As demonstrated by Miller and other authors, such conditions are very favorable to the prebiotic synthesis of organic matter on Earth. In Fig. 1, part C, we also compare these data with the estimation of the age of the first biosignatures of life, which has been recently shifted to 4.1 Gy before the present (56, 57). Considering the direct synthesis of biomolecules on the early Earth and regarding the expectations of a very early reducing atmosphere together with the expectations of very ancient life on Earth, we can assume that biomolecules were probably synthesized on Earth during transformation of the reducing atmosphere. During a time of decreased impact activity, this atmosphere was depleted and transformed to a CO2-rich mixture containing N2 and H2O vapor (58). In this chemistry, formamide can play a role of reactive intermediate. However, we should note that alternative scenario of the theory of exogenous synthesis regards impacts of extraterrestrial bodies not only as a source of shock wave energy and degassed reducing atmospheres but also as a direct source of biomolecules. These assumptions are supported by recent findings of a wide palette of organic substances (59) and biomolecules in chondritic meteorites (60, 61).
Results and Discussion
In the following experiments, stable and unstable products were monitored during and after the exposure of a NH3 + CO + H2O reducing mixture to electric discharge simulating lightning and laser shock wave simulating asteroid impact plasma. In the first step, electric discharge in the mixture NH3 + CO + H2O was investigated by emission time-resolved Fourier transform high-resolution spectroscopy (refs. 62 and 63, and references therein). In the emission spectra depicted in Fig. 2, several species that are very similar to the discharge of pure formamide can be observed (19). In all of the spectra, the most prominent bands belong to CO, CO2, HCN, and HNC excited to high vibration–rotation levels and the unstable but ubiquitous radical ·CN, which can play an important role in the subsequent plasma synthesis of nucleobases (18⇓–20). Stable molecules, such as ammonia, carbon monoxide, acetylene, nitrous oxide, and hydrogen cyanide, were identified by high-resolution infrared absorption spectroscopy during subsequent analysis of discharge products concentrated in liquid-nitrogen trap. The absorption spectra are shown in Fig. 3.
Fig. 2.
Emission spectrum of a reducing atmosphere (NH3, CO, H2O) discharge plasma. Among others, carbon monoxide and the ·CN radical in a wide range of energetic states dominate the spectra.
As the most important finding, discharge treatment of NH3 + CO + H2O led to the formation of a significant amount of formamide and HCN. The formamide absorption band is marked in red in Fig. 3 (details are supplied in Figs. S1 and S2). As discussed in the following section, this result fully corresponds to theoretical predictions of the reactivity of Miller-type mixtures.
Fig. S1.
A portion of absorption spectrum in the region of a carbonyl vibration shows the absorption v4 band of formamide covered with very strong series of water lines. The formamide reference spectrum is shown in red.
Fig. S2.
Absorption spectra of ammonia and carbon monoxide with treatment by an LIDB plasma shock wave. A shows the blank before the irradiation, B supplies the spectra recorded after 15 pulses of the laser without catalytic material, C depicts the spectra after 15 laser pulses in presence of montmorillonite, and D shows spectra of products of nucleobase treatment in discharge plasma.
Opposite to our gas-phase discharge experiments, we also explored the decomposition of solid samples of pyrimidine (adenine) and purine (cytosine) bases in presence of water vapor. The samples were inserted into glass discharge tubes and treated with nitrogen discharge plasma. Similar to the decomposition of formamide or the NH3 + CO + H2O mixture, we detected a large amount of HCN, which is one of the products of formamide decomposition. As seen by comparing of Fig. 3 with Fig. S2 B–D, we detected a similar grouping of products from nucleic base decomposition and NH3 + CO + H2O atmosphere discharge treatment.
Finally, in the following experiments, we exposed a simple binary reaction mixture of NH3 and CO to a plasma shock wave without the presence of a catalyst and in the presence of montmorillonite clay and water. In our previous study, montmorillonite was identified as a plausible catalyst for nucleic base synthesis upon laser shock wave. Fig. S2C shows the composition of the reaction mixture after exposure to the laser shock wave generated in the gaseous media. Comparison of both mixtures to a blank sample is supplied in Fig. S2 A and B. As the most important result discussed in the following section, we observed hydrogen cyanide as a main product of formamide thermal decomposition in both experiments. Additionally, we show that, although we used a very simple mixture of Miller–Urey-type reducing gases (and not directly formamide, similarly to previous experiments), we detected all of the RNA canonical nucleobases—uracil, cytosine, adenine, and guanine—together with urea and the simplest amino acid, glycine. The conclusion of the analysis is shown in Table 1. However, we should note that the sub-parts per million level reached in laser experiments for pyrimidine bases is a threshold detection limit of the used method. Nevertheless, these findings support the idea that a NH3 + CO + H2O atmosphere can substitute for pure formamide (19) and act as a starting environment not only for the formation of amino acids (as shown in previous works) but also of RNA nucleobases. Fig. S3 depicts the chromatograms of all of the canonical nucleobases using a selected mass filter for the typical m/z ratio for each particular base. Typical mass fragments of all of the analytes are clearly identified at the appropriate retention times, as shown in Figs. S4–S6. We also note that we exclude any external contamination of the samples (SI Contamination and blank measurements depicted in Fig. S7).
Table 1.
The results of all of the experiments with laser and discharge plasma treatment of a reducing atmosphere
Fig. S3.
GC-MS detection of nucleic bases in all of the experiments. All samples were analyzed after derivatization by with MTBSTFA. A shows the chromatogram of a blank of the canonical nucleic bases, B shows the chromatogram of the experiment with irradiation of the mixture of NH3 + CO, C depicts chromatogram of the experiment with NH3 + CO + H2O in presence of clay, and D shows the discharge decomposition of NH3 + CO + H2O. E and F depict, respectively, chromatograms of adenine and cytosine glow discharge decomposition upon nitrogen atmosphere in presence of water vapor.
Fig. S4.
The typical mass spectra received using GC-MS for nucleic bases and glycine after derivatization. All the mass spectra are observed exlusively in typical retention time of each particular molecule as shown in Fig. S3 [for nucleobases; Fig. S3A for standards and Fig. S3 B–F for experimental samples) and in the Fig. S5 (for glycine; Fig. S5A for standard and Fig. S5 B–F for experimental samples). A depicts mass spectrum of uracil standard with the typical fragment m/z = 283 compared with uracil mass spectra discovered in the experimental sample; B shows comparison of experimental spectra with adenine-1 standard with the typical fragment m/z = 192; C carries out the similar comparison for cytosine with the typical fragment m/z = 282; D depicts this comparison for the adenine-2 mass spectrum with typical m/z = 306; E depicts this comparison for guanine with typical fragment m/z = 322; and F shows glycine with fragments m/z = 147, 226, and 246. The corresponding structures of typical mass fragments are depicted in Fig. S6.
Fig. S5.
GC-MS detection of the simplest amino acid glycine in all of the experiments. All samples were analyzed after derivatization by with MTBSTFA. A shows the chromatogram of a blank of the glycine standard, B shows the chromatogram of the experiment with irradiation of the mixture of NH3 + CO, C depicts chromatogram of the experiment with NH3 + CO + H2O in presence of clay, and D shows the discharge decomposition of NH3 + CO + H2O. E and F depict, respectively, chromatograms of adenine and cytosine glow discharge decomposition upon nitrogen atmosphere in presence of water vapor.
Fig. S7.
Chromatograms of blank measurements, all of them with a silylation agent, together with a chromatogram of NH3 + CO + H2O discharge products for comparison depicted in A. Nucleobases are observed on the parts per million level in this experiment. B and C show a chromatographic record of a cell for laser shock wave experiment not exposed to plasma and discharge cell, respectively, both washed after 1 d with water; D shows chromatogram of a cell touched with finger without gloves; and E shows a record of water after washing of montmorillonite catalyst. Numbers 1–9 mark manifold products of mutual reactions in derivatization agent.
To help interpret the reactions in the shock wave plasma (4500 K) and electric discharge (650 K) experiments, further ab initio molecular-dynamics simulations combined with enhanced sampling were performed (48). We remark that our simulations proceed without introducing any information about the possible reaction pathway. The simulations are focused on gas-phase systems (two-body collisions) and on understanding the thermally activated reaction mechanisms (fully including anharmonic motions and entropic contributions) pertaining to the ground electronic state. Exploiting these ab initio simulations, we obtain evidence that the CO + NH3 system passes through formamide as an intermediate before transforming into HCN and water in a second step. Starting from pairs of molecules taken from the experimental Miller-like gas mixture (CO + NH3 or CO + H2O), we simulated possible reactions in the direction of the detected products, including formamide (traces in the mixture of NH3, CO, and H2O in the presence of a clay) and HCN (in both experiments), at different temperatures (Fig. 4A).
Fig. 4.
Reaction mechanisms and free-energy profiles (at 650 K in blue and at 4500 K in red; values in kilocalories per mole) from metadynamics and umbrella sampling simulations, for binary reactions between simple Miller-like molecules, leading to formamide (A) and formic acid (B).
The reaction between CO and NH3,
[1]yields formamide, overcoming a free-energy barrier of 52 kcal/mol at 650 K, which increases to 116 kcal/mol at the shock wave plasma temperature of 4500 K. When this is measured in units of available thermal energy kBT, the free-energy barrier is reduced from 40 to only 13, such that the calculations predict fast kinetics at high temperature.
As mentioned above, a significantly larger amount of formamide in the CO + NH3 discharge experiment (650 K) was observed compared with that observed under laser shock wave plasma (4500 K) conditions. At the same time, after very fast synthesis at high temperature, our simulations predict that formamide is significantly destabilized with respect to CO + NH3 at 4500 K, leading to a small observed experimental concentration. Despite the variation in barrier height, the reaction mechanism at the two temperatures appears basically unchanged. Destabilization of formamide leads to its decomposition, and this molecule can then serve again as an unstable intermediate. Following the experimental observation of sizable amounts of H2O and HCN at both 650 K and 4500 K in nearly a 1:1 ratio, we simulated their formation either starting from formamide or directly from the initial Miller mixture:
[2]Starting from formamide, proton transfer leads first to an intermediate OHCHNH species, which then dissociates into water and HCN (Fig. 5A).
Fig. 5.
Reaction mechanisms and free-energy profiles (at 650 K in blue and at 4500 K in red; values in kilocalories per mole) from metadynamics and umbrella sampling simulations, starting from formamide (A) or from formic acid and ammonia (B).
The two consecutive barriers are 42 and 55 kcal/mol at 650 K and only 33 and 36 kcal/mol at 4500 K. Under the latter conditions, HCN and water are very stable compared with formamide, in contrast to the conditions at 650 K; thus, the calculations predict observable amounts of formamide only at the lower temperature. This in turn may explain why HCN was, and formamide was not detected in shock wave plasma experiments (i.e., because it is a necessary, but very unstable intermediate), whereas it was found under milder conditions in the electric discharge experiment (Table 1). Additionally, HCN can be decomposed to the ·CN radical, which was observed by means of emission spectroscopy:
[3]This radical reacts with formamide produced in this high-temperature chemistry. This reaction channel represents an additional sink of this molecule. As described in detail in our previous reports, such reactions lead to the subsequent synthesis of nucleobases, also involving the H· and ·NH2 radicals (19, 21).
Stemming from the strong similarity between the experimental products obtained when starting from the Miller mixture (passing through the formation of formamide) and the experimental products obtained when starting from formamide (formation of the Miller mixture, i.e., NH3 + CO together with HCN), with the creation of nucleobases in both cases (Table 1), we hypothesized that formamide is indeed an obligate intermediate of reactions leading to HCN and further reactions toward nitrogenous bases. It was very interesting that, in a separate benchmark simulation starting from CO + NH3, which was aimed at producing HCN without introducing any information about the possible reaction pathway (SI Methods) in our simulations, we found that the system passes through formamide as an intermediate before transforming into HCN and water in a second step. This unbiased result thus supports our hypothesis of formamide as an obligate intermediate.
We also addressed the formation of formic acid in the solution phase and its role as a possible intermediate toward other molecules, including formamide (Fig. 4B). Starting from CO + H2O, according to the following equation:
[4]the formation barrier increases from 57 kcal/mol at 650 K to 96 kcal/mol at 4500 K. At both temperatures, the barriers are thus comparable with those for the formation of formamide, a situation already observed in aqueous solution (48). Once formed, the collision of HCOOH with ammonia yields formamide and water (Fig. 5B):
[5]with barriers of 54 kcal/mol at 650 K and 48 kcal/mol at 4500 K. Interestingly, in this latter case, the mixture of CO + NH3 + H2O is formed as an intermediate, that is, HCOOH breaks down into CO + NH3 before the formation of formamide:
[6]We did not actually detect HCOOH in any of our experiments with the reducing mixture.
The reactions investigated here have been previously studied using static quantum chemistry calculations, for instance, in ref. 64 (formamide and HCN formation) and in ref. 65 (HCOOH formation). The transition states and barrier heights in the latter works are quite similar to our results at T = 650 K, considering the different treatment of temperature (approximations based on harmonic vibrations in the literature versus molecular-dynamics in our case) and the different quantum-mechanical approximations [B3LYP, G2M, MP2, and CCSD(T) in the literature versus density functional theory–Perdew–Burke–Ernzerhof (DFT-PBE) in our case]. We carefully verified that each reaction pathway was reproduced at least three times in the metadynamics, either in independent or in the same simulations (due to multiple forward and backward transitions). This fact, together with the similarity between pathways found at 650 K and in the aforementioned literature, makes us confident in the robustness of our results.
Link collected : https://www.pnas.org/content/114/17/4306